Detergents in gasoline are different from the regular detergents we use for washing (although not that different to be honest). The role of detergent additives in fuel or even oil, for that matter is a crucial one.
So why are detergents added to gasoline? Detergents are added to gasoline to prevent deposit formation. Gasoline contains lots of impurities and different chemicals that can lead to deposit buildup and detergents help in preventing this buildup.
The detergent additives keep the engine and the engine components free of any deposits. In addition, detergents neutralize the formation of any acids in the fuel.
While that’s great, let’s dig in-depth into the role of detergents and how exactly they work.
What are detergents here?
Detergents are additives used to clean and neutralize the impurities in the fuel which otherwise would have formed deposits.
These impurities if not for detergents would result in deposit formation all over the engine. And deposits attract deposits. Before you know it, the engine components will be full of carbon deposits and sludge all over.
That’s why detergents are important additives in fuel. Heck, even engine oil needs detergents to avoid deposit formation.
As for why gasoline needs detergents? Gasoline contains lots of impurities and different chemicals that lead to deposit buildup and engine gunk. Detergents are added to gasoline to avoid such deposit formation.
The impurities in gasoline come from many different sources.
- First, the base stock which is crude oil itself is impure with a lot of contaminants and chemicals.
- Next, the refining process does not help in reducing impurities. The gasoline obtained actually contains a lot of other chemicals rather than being ridden the impurities. The molecular variability of refined gasoline is high.
- In addition, the refiners and retailers add many chemicals to gasoline. These chemicals range from ethanol to octane enhancers or other additives like anti-knock agents and fuel dyes.
- Lastly, while these additions help in their specific functions, they are still impurities contributing to deposit formation.
That’s why the solution to prevent such deposit formation in the engine is to add detergent additives to the gasoline.
Role of detergent additives in gasoline

Detergent additives perform two main roles in gasoline.
First, the detergent additives keep the engine and the engine components free of any deposits.
These engine components include sensitive components like the fuel injector nozzle and intake valves. Both of which are essential for the air-fuel mixture to enter the engine.
If there are deposits in these components, the deposits can absorb or block the fuel from entering the engine. As a result, the fuel combustion process gets inefficient and you will soon start to notice reduced gas mileage as well as engine power.
Second, detergents neutralize the formation of any acids.
Detergents are primarily alkaline in nature. Any acids formed within the gasoline due to contamination and the heat are neutralized by these detergent additives.
The detergents ensure that any impurities and foreign objects in the gas stay in suspension and do not allow them to form acids.
So having detergent additives is better than not having them, be it in fuel or in oil.
This functional aspect of the detergents makes detergent additives more attractive to be used in gasoline. Most modern vehicles – both cars and motorcycles perform much better with detergent additives in the gasoline used.
How detergents work in gasoline
Detergents are usually made of nitrogen-containing compounds called amines. They consist of two main components:
- a long hydrocarbon tail
- a polar head
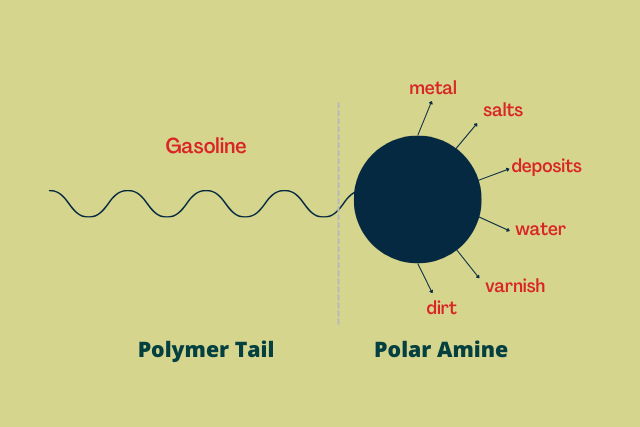
The long hydrocarbon tail helps the detergent to be soluble in gasoline, or even other fuel or oil for that matter.
The polar head, on the other hand, attracts and keeps impurities from depositing. These impurities range from water to metals to varnish to dirt.
The polar head, which is made of amines, has the ability to attract a diverse range of impurities and keep them afloat. Thus, preventing these impurities from depositing, combusting, or wearing down the engine components.
The detergents are usually prepared by starting with a polymer of polybutene first. A polar function is grafted then on the polymer, such as an amine by means of a polar linking group. In some cases, the polymer backbone may contain oxygen well to improve the detergent’s effectiveness.
The output formed from such a process will lead to an effective detergent additive – keeping sludge, soot, oxidation products, and other deposit precursors from forming deposits onto and harming key engine parts.
List of detergents commonly used
So what are the common detergents used in gasoline then?
Here are the three main types of detergents added to gasoline to prevent deposit formation.
- Polybutene amine (PBA): PBA is the first deposit-control additive type used in gasoline. The F-310 additive of the 1970s in Chevron branded gasoline was made of PBA. Although other hydrocarbon amines have entered the picture, polybutene amine detergents are still popular and relevant even today.
- Polyether amine (PEA): Initially developed as a filler for plastic bottles, later found its popular use as a detergent additive in automotive. It is light and easy in its application with no harmful effects on the environment acting as a big plus. PEA’s major application actually is in diesel engines rather than gasoline.
Techron is a popular polyether amine detergent developed by Chevron to clean up grimy deposits. Other variants include polyethylene terephthalate (PET) and polyethylene glycol. - Polyisobutylene amine (PIBA): PIBA is steadily growing in popularity as the choice for detergent additives in gasoline. Along with acting as a detergent, PIBA is also known for its anti-corrosion properties (PIB to be specific, the amine part is more to attract impurities).
Other additives added to gasoline
Apart from detergents, there are other additives added to gasoline to enhance different characteristics. These additives include:
- Anti-knock additives: reduce engine knocking and increase the fuel’s octane rating by raising the temperature and pressure at which auto-ignition occurs.
- Corrosion inhibitors: used to protect engine components from corrosion damage. Also helps in preventing corrosion and deterioration of fuel storage and distribution systems.
- Antioxidants: inhibit oxidation (duh!). This prevents any free radical chain reactions involved in hydrocarbon oxidation. Basically no unwanted free radicals or chain reactions in gasoline.
- Oxygenates: are added to reduce carbon monoxide and soot that is created during the burning of the fuel. Typically alcohols and ethers are used as oxygenating additives in gasoline.
- Other additives: include anti-misfire additives, spark-aid additives, anti-icing additives, drag reducing agents, and dyes.
To summarize
Detergents are additives used to clean and neutralize the impurities in the fuel which otherwise would have formed deposits.
Gasoline contains lots of impurities and different chemicals that lead to deposit buildup and engine gunk. That’s why detergents are added to gasoline to avoid such deposit formation.
The impurities in gasoline come from various sources – from crude oil itself, the refining process, refiners and retailers, etc.
The detergent consists of two main components – a long hydrocarbon tail and a polar amine head.
The long hydrocarbon tail helps the detergent to be soluble in gasoline, or even other fuel or oil for that matter. The polar head, on the other hand, attracts and keeps impurities from depositing.
The three main types of detergent compounds used are – polybutene amine (PBA), polyether amine (PEA), and polyisobutylene amine (PIBA).
Apart from detergents, other gasoline additives such as anti-knock agents, corrosion inhibitors, oxygenates, and antioxidants are also added to gasoline.
